-
Psychological Stress in Children: Effects on the Immune Response
By Roberta Attanasio Stress is part of life — but while a little bit of it (good stress) may keep us active and alert, and sometimes even motivate us, the long-term type (bad stress) can have negative effects on our health. Elevated blood pressure and heart disease are just some examples of the so-called “stress-related diseases”. In addition to good stress and bad stress, there is another type of stress — toxic stress. Professor Pat Levitt defines toxic stress as “a term used by psychologists and developmental neurobiologists to describe the kinds of experiences, particularly in childhood, that can affect brain architecture and brain chemistry. They typically are experiences that are…
-
Sex Differences in the Immune Response to Vaccines
By Roberta Attanasio Women and men respond differently to infectious microbes and vaccines – it is said, indeed, that the immune system of women is stronger than the immune system of men. Stronger or weaker, one thing is certain – men and women are not the same in terms of immune response. A few years ago, the journal Lancet Infectious Diseases published “ The Xs and Y of immune responses to viral vaccines” – a comprehensive article that clearly shows how the biological differences between sexes influence the immune response to vaccines, as for example the influenza, yellow fever and hepatitis vaccines. There are not many published studies on the…
-
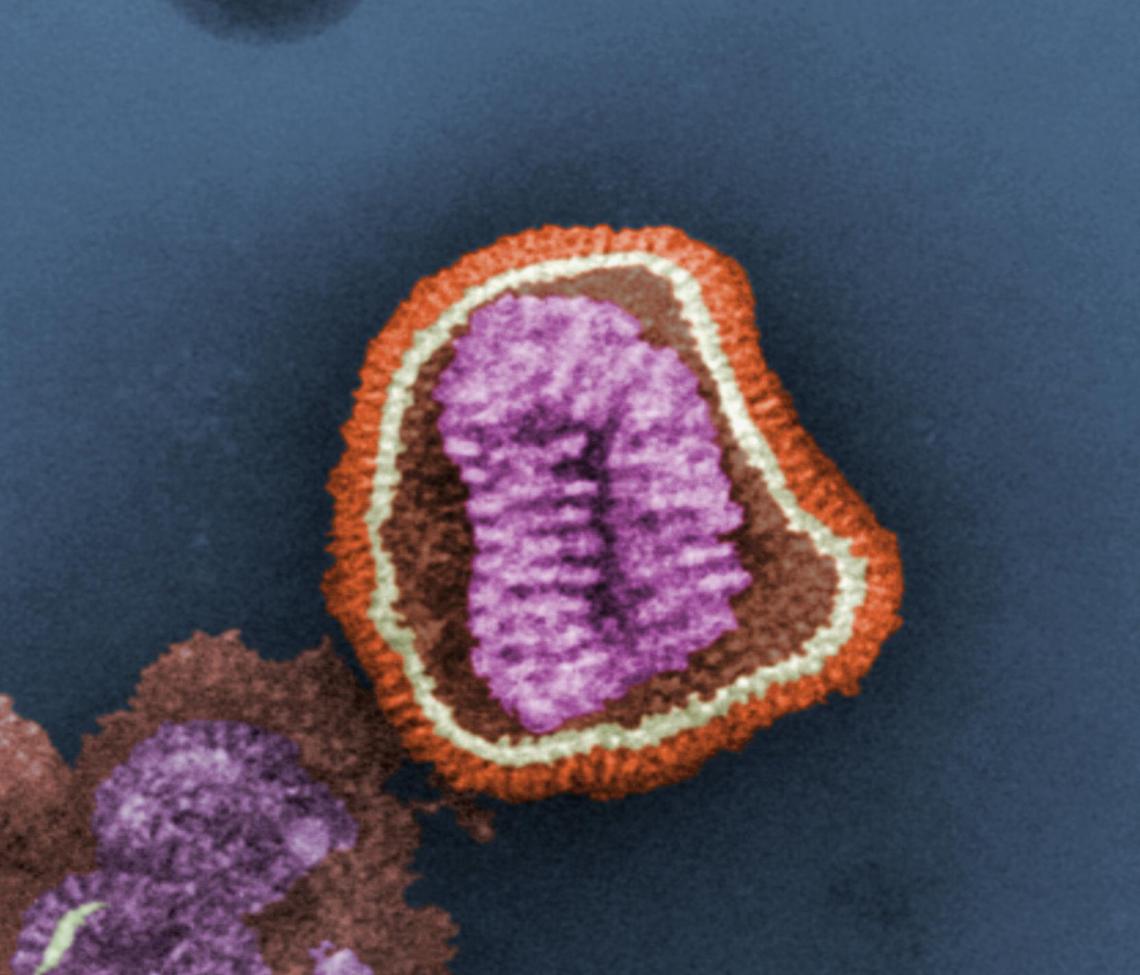
H7N9 Influenza Virus: Ethnicity and Protection from Infection
By Roberta Attanasio In March 2013, a new flu virus — the H7N9 — was identified in China. By early May, before retreating and disappearing, it had infected 131 people and killed 26 of them. However, less than two weeks ago (January 17), the New York Times reported that “China is disclosing a steadily growing number of cases of H7N9 bird flu, including four more cases announced on Friday, reviving concerns among health experts that the disease may be spreading and could pose a further threat as the world’s largest annual human migration begins ahead of Chinese New Year.” The H7N9 virus is a “reassortant” — it includes combined elements from three…
-
The Great Global Die-Off: Frogs and Lymphocytes
By Roberta Attanasio Frogs and other amphibians – salamanders and caecilians – have been declining worldwide during the past few decades at an alarming rate. According to a June 2012 assessment by the International Union for the Conservation of Nature and Natural Resources (IUCN), about 41 percent of amphibian species are at risk of extinction, and some are already extinct. Like many other inhabitants of our planet, amphibians have been hit hard by climate change and habitat loss – and not only. Amphibians have also been decimated by the spread of chytridiomycosis, which is defined by the IUCN as the single most devastating infectious disease of vertebrate animals. In a…