-
Prenatal Exposure to Pollutants: Influence on the Immune Response
By Roberta Attanasio The development of the immune system during fetal and neonatal life is negatively influenced by exposure to toxic chemicals, resulting in compromised immune function later in life. An example is fetal exposure to arsenic, which has deleterious effects on the immune response to influenza virus infection in adulthood. Now, results from a new study provide additional evidence for the role that exposure to toxic chemicals early in life plays in shaping the immune response to the influenza virus. The study (by researchers at the University of Rochester) focused on a mouse model and the chemical 2,3,7,8-tetrachlordibenzo-p-dioxin, or TCDD for short. TCDD, a known carcinogen, is a persistent environmental contaminant…
-
Sex Differences in the Immune Response to Vaccines
By Roberta Attanasio Women and men respond differently to infectious microbes and vaccines – it is said, indeed, that the immune system of women is stronger than the immune system of men. Stronger or weaker, one thing is certain – men and women are not the same in terms of immune response. A few years ago, the journal Lancet Infectious Diseases published “ The Xs and Y of immune responses to viral vaccines” – a comprehensive article that clearly shows how the biological differences between sexes influence the immune response to vaccines, as for example the influenza, yellow fever and hepatitis vaccines. There are not many published studies on the…
-
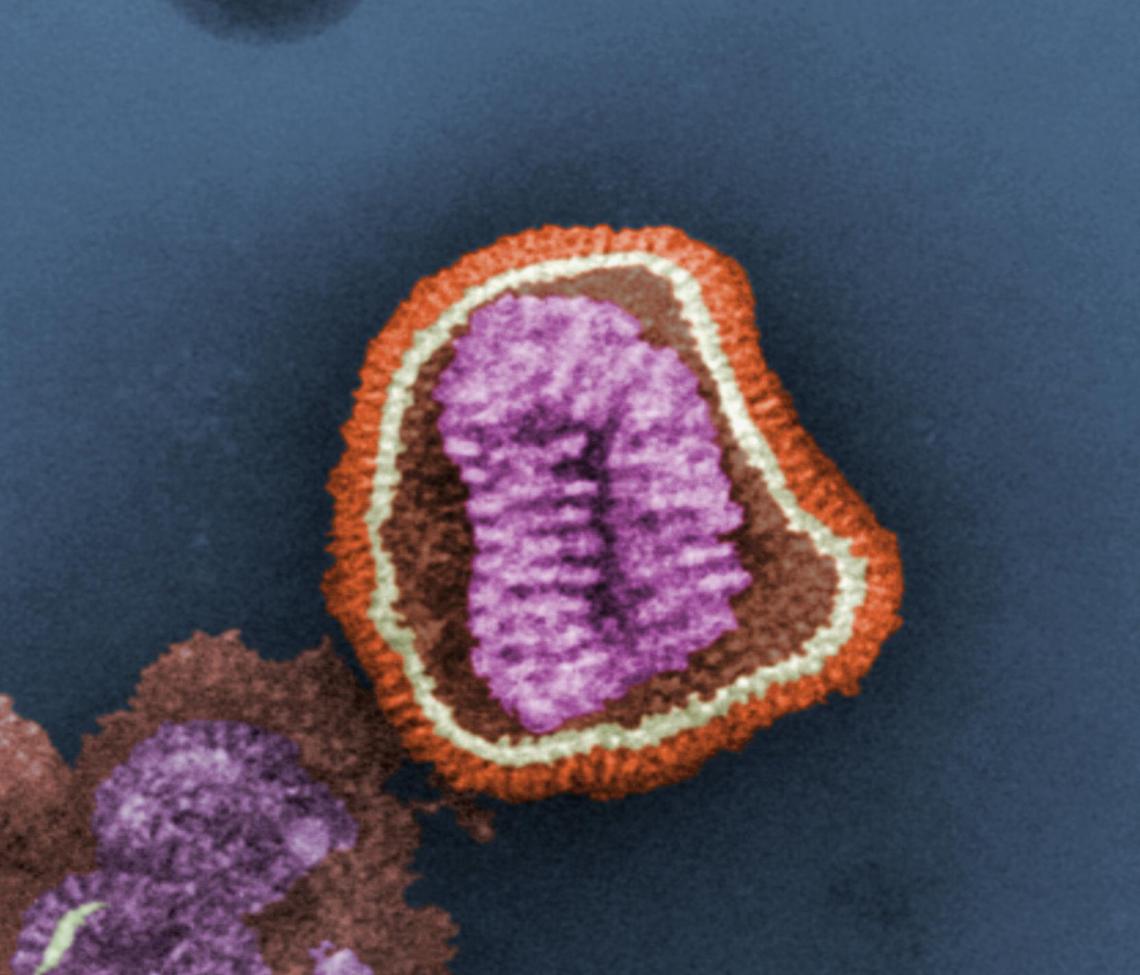
H7N9 Influenza Virus: Ethnicity and Protection from Infection
By Roberta Attanasio In March 2013, a new flu virus — the H7N9 — was identified in China. By early May, before retreating and disappearing, it had infected 131 people and killed 26 of them. However, less than two weeks ago (January 17), the New York Times reported that “China is disclosing a steadily growing number of cases of H7N9 bird flu, including four more cases announced on Friday, reviving concerns among health experts that the disease may be spreading and could pose a further threat as the world’s largest annual human migration begins ahead of Chinese New Year.” The H7N9 virus is a “reassortant” — it includes combined elements from three…